|
What are linear equations? Linear equations generally will contain an x and y variable in an equation, which contains an equal sign (=). In this equation, you can input an x value and solve for the y value to plot a point on the coordinate plane. The coordinate plane is essentially two number lines, one vertical and one horizontal, that creates a two dimensional space in which points and lines can be graphed. The horizontal axis is the x-axis and the vertical axis is the y-axis. In a linear equation, for any number, x, you can plug it into the equation and solve for y. Then, on the coordinate plane, at the x value and the y value, a point can be plotted. For linear equations, the result is a line on a graph, but how do we get a line on the graph? With linear equations, you can choose two x values and solve for y. Then, plot the points on the graph and connect them with a straight line. Now, to get the y value for any x value, you can simply find the x value on the x-axis and then find what the y value is for that x value! This may still be confusing, so let’s look at an example: Linear equation: y = 2x + 1
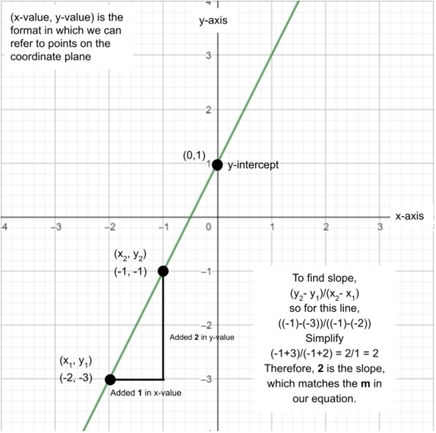
This form is called slope-intercept form. Many other forms of the linear equation exist, but we will focus on just this one. This form can be put as y=mx+b. In this, m is the slope and b is the y-intercept. The x and y are both variables. To calculate slope, find two points in the line by plugging any two x values into the linear equation. In the image, we plugged in -2 and -1 as our two x values. This results in:
y = 2(-2) + 1, so y = -3 → (-2, -3) y = 2(-1) + 1, so y = -1 → (-1, -1) Although we know just looking at the equation that the slope of the line is 2, we can also calculate it using the equation (y2- y1)/(x2- x1). Looking at the calculations shown on the image, we know that the slope will be 2. Let’s practice! We are given points (1,4) and (3,-2). Find the slope. Solution: Let’s make (3,-2) the (x2, y2) and make (1,4) the (x1, y1). Now we can plug the value into the equation, (y2- y1)/(x2- x1). Therefore, (y2- y1)/(x2- x1) → ((-2) - 4)/(3 - 1) = (-6)/(2) = -3 Slope = -3 Now, we can also find the y-intercept by plugging in a single point and the slope since we know that the slope is m. y = -3x + b 4 = -3(1)+b 4 = -3 + b (now we will add -3 to each side to solve for b) 7 = b Therefore, our complete equation for the line containing the points (1,4) and (3,-2) is y=-3x+7.
0 Comments
What is Force?
Force is a term in physics used to describe a push or pull on an object. A force can make an object go faster, slow down, remain in shape, or change shape. For example, when you push a shopping cart at the grocery store, you are putting force on the cart which is causing it to get faster and move with you as you are walking! How is Force Measured? Force is measured in Newtons, abbreviated “N” for short. A newton is the amount of force needed to accelerate (move) one gram of mass by one centimeter per second squared. Force can also be measured in dynes and pound forces. What is Acceleration? Acceleration is the amount of change of an object’s velocity (speed). For example, when you step on the gas pedal in your car, your car speeds up, and when you step on the brake, your car slows down. When your car speeds up and slows down, it is accelerating! How is Acceleration Measured? The standard unit of measure for acceleration is m/s^2, where the speed is in meters per second and time is in seconds. Since acceleration has both a magnitude (size) and a direction, we call it a vector quantity. How are Force and Acceleration Related? Force and acceleration can be related to each other through this equation: F = m * a F = amount of force applied to object m = mass of object a = acceleration of object Source: https://www.ducksters.com/science/physics/ When you look at the periodic table, it may look scattered, almost unorganized. You may wonder, why isn’t it simply a rectangle, and what are all those letters and numbers on it?
The shape of the periodic table is determined by the elements’ atomic number, valence electrons, and the number of energy levels they contain. Reading from left to right, we will notice that the number at the very top of the box, called the atomic number, increases with each following element. The atomic number is also what defines an element because the atomic number is equal to the number of protons in that element. In any element, the number of protons, positive particles in the nucleus of elements, is what makes it that specific element. Therefore, if we have an element with three protons, we know it is lithium because the number of protons equals the atomic number of the element. The valence electrons and chemical properties of an element determine its column on the periodic table. Valence electrons are the electrons on the outermost energy level of an atom. All atoms have energy levels, starting from the innermost that holds two electrons, the next level with eight, and each following level having a specified capacity for electrons. Therefore, on the leftmost column of the periodic table, all those elements have only one valence electron, meaning their outermost energy level only has one electron. However, in the rightmost column, all the elements have the maximum valence electrons for the element. However, this gets more complex when we go lower in the periodic table, so another way to recognize groups, or columns, is by their similar chemical properties. Since the elements in the same column have the same number of valence electrons, they have similar reactivity among other chemical characteristics. The number of energy levels of an element determines which row, or period, it belongs to. In the first period, we have hydrogen and helium, both of which only have one energy level, therefore placing them in the very first row. However, in the next period, all the elements require two energy levels to hold all their electrons. From this , we know that if we were to take the element magnesium (atomic number 12), that it would have three energy levels since it’s in the third period! Now let’s do a little trivia! Find calcium (Ca).
Matter is all around you. Everything around you is matter. A person. An apple. A table. Water. Paper. The list goes on and on. Matter means anything that has mass and volume. But this opens doors to other questions. What is mass? What is volume?
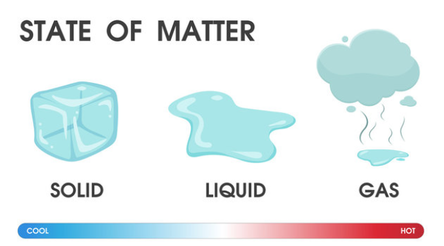
Mass is the amount of matter (or “stuff”) inside of an object. Think of it this way. A balloon has very little mass. But a building has a lot of mass. Be careful though, there is a difference between mass and weight. Mass measures the “stuff” inside of an object. Weight refers to the measure of gravity’s pull on an object. In other words, it determines how heavy something is. This is why you would weigh much lighter on the moon because the moon has less gravity. Volume refers to the amount of SPACE that something takes up. When we say something is big, small, tiny, long, all of these words describe something’s VOLUME. For example, a coin has a small volume because it takes up only a small amount of space. But, a textbook has a larger volume because it takes up a lot of space. Different states of matter fill spaces in different ways. For example, water fills volume in a different way than a textbook does, right? So, what are the different states of matter? There are three basic states of matter: solid, liquid, and gas. Solid is a state of matter that keeps its shape. Think about a textbook or a table. Liquid is a state of matter that takes the shape of its container. Think about water in a container. It takes the shape of the container, right? Gas is a state of matter that fills its container. Think about water vapor or steam or even air all around us. It doesn’t have a shape like water, but it fills the entire space around! Let’s take water as an example. Water can be in solid form as an ice cube! Also, water can be in liquid form, which is what we are most used to seeing! But, water can also be seen in gas form, which you can see if you were to heat or boil water. The water evaporates and becomes water vapor, the gaseous form of water. “Genetics is the science of studying how living things pass on certain characteristics to other generations.” Genetics is something we inherit from our previous generation and it is passed on for multiple generations. Is your hair curly or straight? Is your eye color brown or blue? Are you tall or short? The reason behind the answers to these questions is genetics! They have been passed on to you from your ancestors, which is why children in a family often look like one of the parents.
Although many people contributed to the discovery of DNA and starting the study of genetics, the first person credited with the discovery is Gregor Mendel. He conducted an experiment by crossing pea plants, short and tall to see what kind of children they would create to observe how their visible physical traits are passed down. At first, he guessed that by crossing a short and tall pea plant, he would get a blend of the two and would get the average height of the two plants. But, he discovered that two genes were given to the offspring and that the offspring were all, therefore establishing dominant and recessive genes. Dominant genes are those that can mask another gene if paired with a recessive gene because they are “dominant.” Therefore, since the tall plants were dominant and the short plants were recessive, it made sense that all the offspring were dominant. The offspring created by the cross of these tall and short plants are called the F1 offspring or first-generation offspring. Another cool fact about dominant genes is that even if they are paired with a recessive, an organisms’ physical and visible traits would be that of dominant genes. When a dominant gene is paired with a recessive gene, it is called heterozygous because it is a mix of two different genes. Contrastingly, if a dominant gene is paired with another dominant gene or a recessive gene is paired with another recessive gene then it is called homozygous because they are two of the same types of genes. Interested in learning more about genetics and how passing on traits works? Check this out: https://www.neok12.com/Genetics.htm Sources: https://science.lovetoknow.com/genetics-kids https://eschooltoday.com/science/genetics/what-is-genetics-for-kids.html |
AuthorsNavya Ramakrishnan, Aishwarya Sudarshan, Snaeha Shriram, Ananya Muralikumar Archives
April 2021
Categories |

 RSS Feed
RSS Feed
